The Role of Contract Research Organizations in Modern Pharmaceutical Research

Introduction In the landscape of modern clinical research, Contract Research Organizations (CROs) have emerged as key players in accelerating pharmaceutical innovation. As clinical trials grow increasingly complex, time-consuming, and resource-intensive, CROs provide indispensable support across all stages of the drug development process—from trial design and regulatory submissions to patient recruitment, data management, and post-marketing surveillance. […]
Impact of social media and patient communities: How do these platforms transform recruitment and engagement in clinical trials

Introduction Social media plays an increasingly important role in clinical research, particularly in facilitating patient recruitment and enhancing their engagement throughout trials [1]. Its ability to reach a wide audience and promote interaction between researchers and participants makes it a valuable tool. Additionally, it helps expand the diversity of patients involved in clinical studies, a […]
The role of Clinical Research Coordinators (CRC) and Clinical Research Nurses (CRN) in clinical trials

Introduction Clinical trials are fundamental processes in the development of medical treatments. They require rigorous management and strict adherence to protocols to ensure both the scientific validity of the results and the safety of the participants. Among the key players in these studies are Clinical Research Coordinators (CRC) and Clinical Research Nurses (CRN), two professionals […]
The impact of antimicrobial resistance in Clinical Research and the Pharmaceutical Industry

Introduction Antimicrobial resistance is a global public health issue of unprecedented severity. It is defined as the phenomenon where a microbe evolves to become more or fully resistant to antimicrobials that previously could treat it. Antimicrobials include antibiotics, which kill or inhibit the growth of bacteria. As a result, infections that were once easily treatable […]
The Essential Collaboration: The Role of Principal and sub-Investigator(s) in Clinical Trials

Introduction Clinical trials are a fundamental part of the process for developing new medications and medical devices, ensuring their efficacy and safety before they reach the market. The Principal Investigator (PI) is at the heart of these trials and is responsible for overseeing the study at the investigative site. However, the PI does not work […]
Keys to Effective Quality Management: Principles and Pitfalls to Avoid

Introduction In a world where quality has become a major issue, the evolution of practices is striking. Just as the restaurant industry had to transform its delivery services to meet growing customer expectations, the field of clinical trials has undergone a similar transformation in its pursuit of excellence. Quality Management (QM), is defined as “the […]
Gene therapy trials: A new era of personalized medicine

Introduction Gene therapy, once seen as a distant promise, is now at the heart of a medical revolution, marking the advent of a new era: that of personalized medicine. Unlike traditional one-size-fits-all treatments, personalized medicine is based on the idea that everyone is unique and that treatments should be tailored to their specific genetic profile. […]
Psychiatric clinical trials: addressing methodological challenges

Introduction Psychiatric clinical trials are vital to advancing the treatment and understanding of mental health disorders. This emphasizes the need for biomarkers to enhance psychiatric treatments, particularly for disorders like schizophrenia and depression, where identifying specific subtypes could lead to more targeted and effective therapies [1], but they often face serious methodological challenges that can […]
Clinical clues to caring for aging, complex patients

Introduction The increase in multimorbidity among elderly patients presents a major challenge for healthcare systems, especially within the context of clinical trials [1]. As the population ages, the complexity of medical care rises, necessitating specific adaptations in the design and implementation of clinical trials to ensure relevant and applicable results for this heterogeneous population. Identifying […]
The future of cancer treatment: Insights from immunotherapy trials
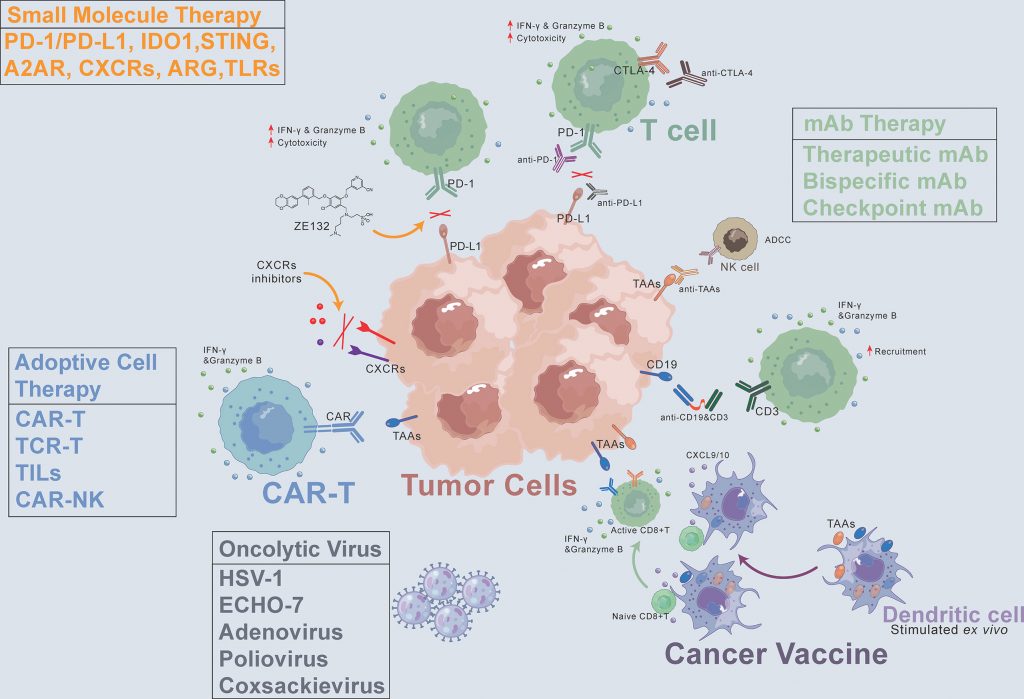
INTRODUCTION Cancer remains one of the most significant challenges in modern medicine, affecting millions of lives globally each year. , with an estimated 1.9 million new cases and 609,360 deaths in the United States in 2022 alone, according to the American Cancer Society’s Cancer Facts & Figures 2022 report [1]. his complex disease encompasses a […]